Proving the Plausibility of Readings
To prove that a measurement result is correct and to exclude or recognize potential error sources, many testing equipments and procedures are available. Especially in the analysis of wastewater and drinking water, legal regulations often explicitely prescribe the requirements or forms of self-checks that have to be completed. In manufacturing industries, service and research laboratories, standard procedures of operation (SOP) for internal quality control and device checks are common. This includes mainly:
- Installation of the instrument by “Installation Qualification” (IQ)
- Geräteüberprüfung
- Assurance of Measurement Plausibility
1. Installation of the Instrument
Usually the installation is defined by a company specific “Installation Qualification” (IQ) procedure . The purpose is to make sure that the instrument is suitable for the given requirements and has the appropriate specifications. Frequently, the IQ is linked to a subsequent „Operation Procedure“ (OP), which can be similar to a „ basic operation training“.

2. Instrument Check
Most instruments carry out a self-check upon turning-on: Depending on the instrument, e.g. the lamp is checked and a wavelength calibration is carried out.
For additional re-examination in daily routine use, many other testing equipments suitable for different instrument types are available:
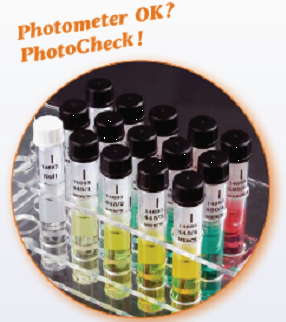
Certified colour solutions like photoCheck® are for filter and spectrophotometers of the photoLab® Series as well as for the portable colorimeters of the photoFlex® Series. photoCheck® is an easy-to-handle testing equipment for many applications. The set is made up of 12 coloured solutions, to be measured at 3 wavelengths with 4 different absorbance levels. They prove photometric accuracy and wavelength accuracy.
Especially for spectrophotometers, there are a lot of highly sophisticated tools, reaching from glass to liquid filters provided by different manufacturers for testing wavelength accuracy, stray light, reproduceability etc. Many meters, like WTW’s photoLab® 7600 UV-VIS are supporting the AQA tools by specific and easy to handle menus.
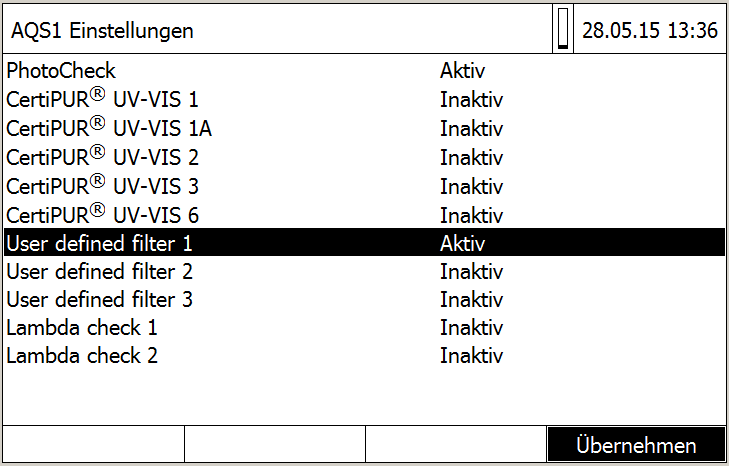
Image 2:
Selection of AQA testing equipments in the user menu of photoLab® 7600 UV-VIS
3. Assuring correct measurement results
A. User administration and periodical monitoring intervals
Many lab meters allow to set intervals for tests with different testing tools and at different test levels. The programme automatically asks for the appropriate standard solution or performs an instrument self-check using the specific testing equipments, e.g. liquid or glass filters.
In bigger companies, the execution of the prescribed tests and checks is usually supervised by an administrator. Spectrophotometers like the photoLab® 7000 Series allow operational level settings for either administrator, user or guest access, thereby ensuring the company’s quality definitions.
Image 3:
Sample test report of an AQA test with photoCheck® and photoLab® 7600 UV-VIS
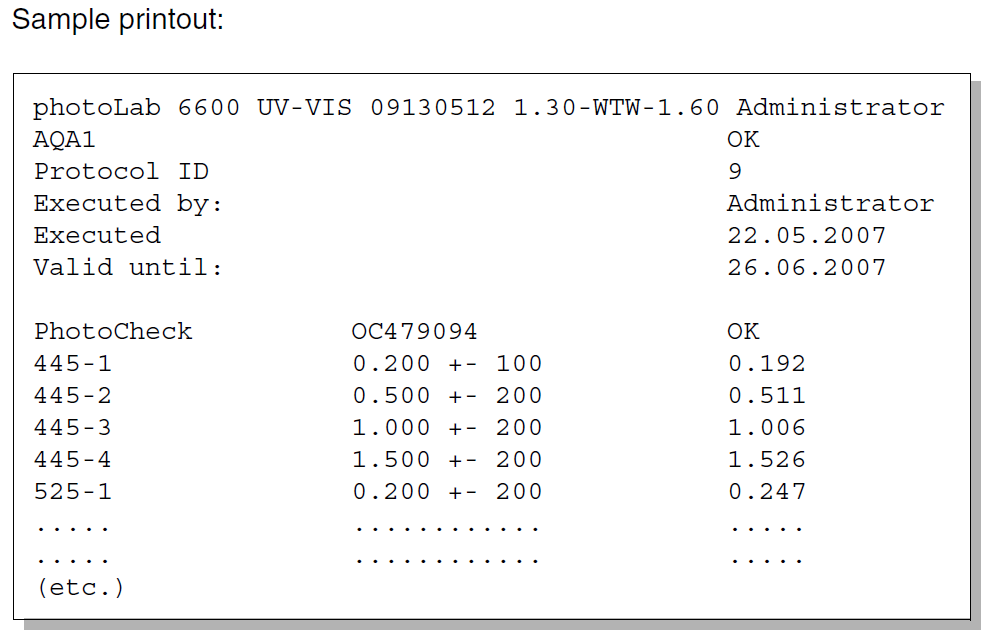
If the AQA system is activated, the measurement results are marked with a AQS identification. At the end of the AQA interval, the settings either do not allow any more measurements at all, or allow only measurements without an AQA identification. The latter is the better option in many cases, since otherwise the instrument is blocked for ongoing measurements.
B. Pipette Check
Pipettes always should be handled with care. A correct upright handling position prevents the contamination of the pipette’s inner walls by traces of former samples. By maintenance and periodical pipette volume check with an analytical balance or a pipette testing equipment such as PipeCheck®, errors can be avoided that might derange the complete measuring system. In the worst case, the contamination might even disturb the chemical reaction in the cuvette.
C. Control Standard Solutions
Performing a test with control standard solutions of default concentrations within the tolerance limits are the easiest way to check the entire system for accuracy and plausibility: If you find the correct value of the control standard within the given tolerance range, you can assume the photometer and the test kit are OK.
For the sample measurement itself, at least a duplicate reading is required to recognize outliners.
All results – samples and control standard – are an excellent verification of a correct measurement system. Wrong readings of the standard solutions are an evidence of a systematic measuring errors and support error investigation, e.g. by spiking.
D. Matrix Check or Spiking
Adding a defined amount of standard solution, the reading must increase accordingly: if not, there is a disturbance in the sample matrix, leading to wrong results.
4. Definition of Errors
Source: Operating Instructions of photoLab® S12, Part 1: General Information (www.wtw.com)
As a matter of principle, measurement results may be defective. This applies equally to standardized methods of analysis (reference methods) and to routine analysis. The discovery and the minimization of errors must be the objective here. A distinction is made between systematic errors and random errors.
Systematic errors are present when all the results of an analysis deviate from the true value by the same algebraic sign. Examples here include: a wrong sample volume, a wrong pH, a wrong reaction time, a sample matrix influence, etc.
- Systematic errors thus affect the accuracy of the method of analysis.
- Accuracy = Deviation of the measured concentration from the true concentration
Random errors manifest themselves in the form of a wide range of deviation of the results of a given sample. These can be kept to a minimum by ensuring good operating techniques and multiple determinations with calculation of the mean value. Random errors make the result of the analysis unreliable; they influence the precision.
- Precision = Dispersion of the results among each other
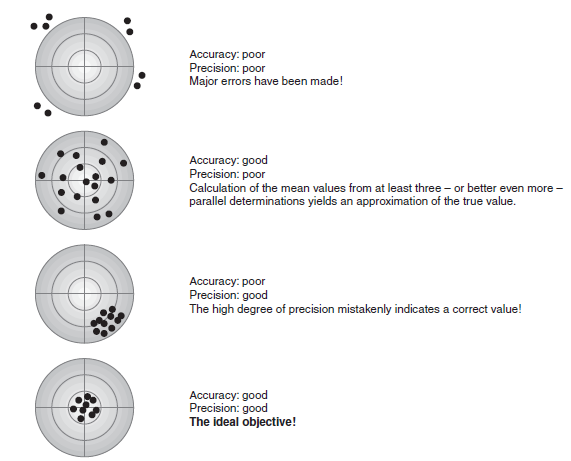
The following diagram illustrates the aspects of accuracy and precision:
Here you can download the whole article as a PDF:
