What is sugar?
 Sugar molecules are made up of three elements: carbon, oxygen and hydrogen. Being of that elemental makeup means sugar belongs in the wider chemical group known as Carbohydrates. The correct chemical term for a sugar is saccharide and there are hundreds of different types of saccharide molecules.
Sugar molecules are made up of three elements: carbon, oxygen and hydrogen. Being of that elemental makeup means sugar belongs in the wider chemical group known as Carbohydrates. The correct chemical term for a sugar is saccharide and there are hundreds of different types of saccharide molecules.
There are many different sugars. The common ones most people have heard of are sucrose, glucose and fructose. Glucose, fructose and sucrose are found organically in fruit and several vegetables. Lactose is found in dairy products and maltose is present in germinating grains.
Less common examples include mannose, and sorbitol – the latter found in potato starch and fruits including apples and pears.
Sugars are formed in nature by the chemical combination of carbon dioxide and water in a complex variety of permutations. Sugars are classified according to their physical (molecular) structure. Their basic structure is formed by carbon and oxygen atoms joined in three, four, five or six-membered rings (known as triose, tetrose, pentose, hexose etc). These rings can themselves be joined together in a daisy chain-like fashion to give the following classification:
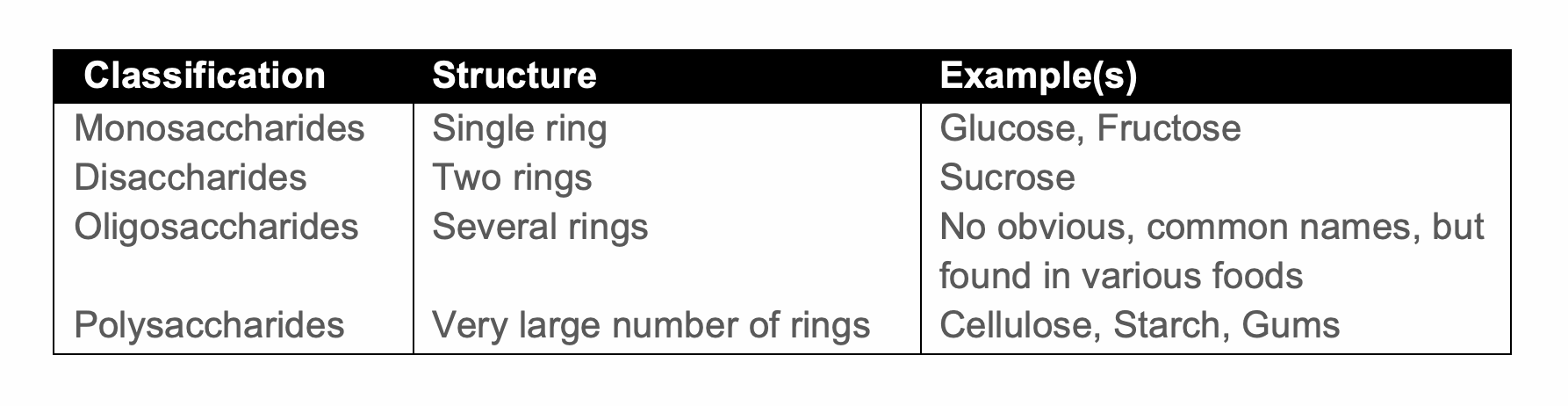
A further complication of sugar molecules is that they are invariably ‘optically active’. This means that the same molecule can co-exist in two or more physical forms (e.g. non-superimposable structures), this is known as chirality. Hence the additional classification: D-, L- forms. This prefix denotes the direction in which polarised light is rotated:
D = dextrorotatory (right hand or clockwise)
L = Laevorotatory (left hand or counterclockwise)
Historically, D-Glucose was called Dextrose and L-Fructose was called Levulose.
For something that most people tend to think of as the thing they stir into their coffee, or sprinkle onto their cornflakes in the morning, it’s all quite complex, isn’t it? – and this is the simple version!
So, let’s look a little deeper…
Sugar Inversion
Sucrose is chemically unstable when dissolved in water. The disaccharide molecule breaks in half to form two monosaccharide units: one molecule of glucose and one molecule of fructose. One molecule of water is ‘consumed’ in this reaction as follows:
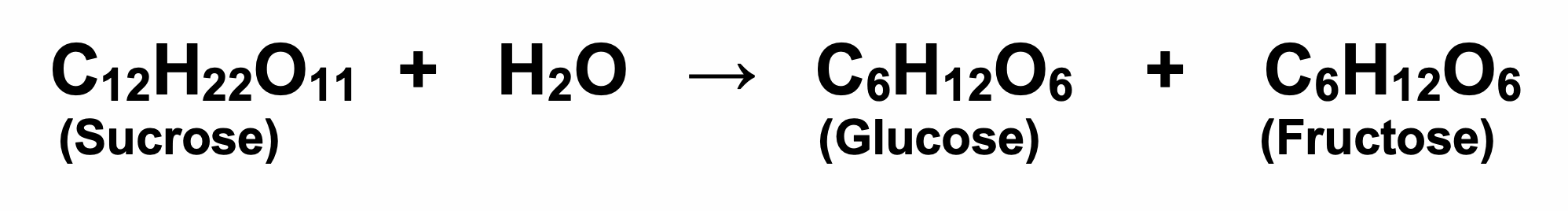
The resulting 1:1 mixture of glucose and fructose is called invert sugar, and the chemical reaction is called inversion. In water the reaction is very slow (taking weeks to achieve). At higher temperatures and in the presence of acid(s) the reaction speeds up considerably. Consequently, when cola drinks are made from sucrose, the dissolved sugar inverts quite quickly because the recipe contains phosphoric acid. Similarly, drinks containing citric acid or ascorbic acid (vitamin C) will also invert quite quickly.
Looking at the above chemical reaction, the weight of dissolved sugar increases (by one water molecule) as the reaction proceeds. At 100% inversion, there is an increase of about 5.3% in the weight of dissolved solids. This accounts mainly for the change (increase) in both density and refractive index of the solution (in this instance, a beverage) when inversion takes place. There is an additional effect: the intrinsic ‘refractivity’ of invert sugar is different from that of sucrose and so the observed change is not simply a linear amount based on the change in weight of dissolved solids. Similarly, the density also does not change in a linear fashion.
Inversion is accompanied by a change in optical rotation. Each of the three sugars has a different specific rotation:
|
Sugar
|
Specific Rotation
|
|
Sucrose
Fructose
Glucose
|
+66.5°
-92°
+52.5°
|
Inversion is accompanied by a swing from positive to net negative rotation because of the overriding negative rotation from the fructose. Because of this effect, polarimetry is a very convenient way to measure the rate of inversion or the degree of inversion in partially inverted solutions.
Practical Uses of Optical Rotation
The use of optical rotation (specific rotation when considering tube length and concentration) is commonplace in general industry as a tool for checking incoming sugars to ensure purity, (i.e. sample spoilage or whether or not adulteration has occurred during transport and or storage) as well as for payment within the sugar industry itself.
Additionally, checking the percent inversion of finished goods is also possible when knowing the start and end points of the chemical reaction for the sample under test.
Bellingham + Stanley’s ADP Polarimeters and ADS Saccharimeters include an inversion and %Inversion Method to help users manage their sample testing efficiently.
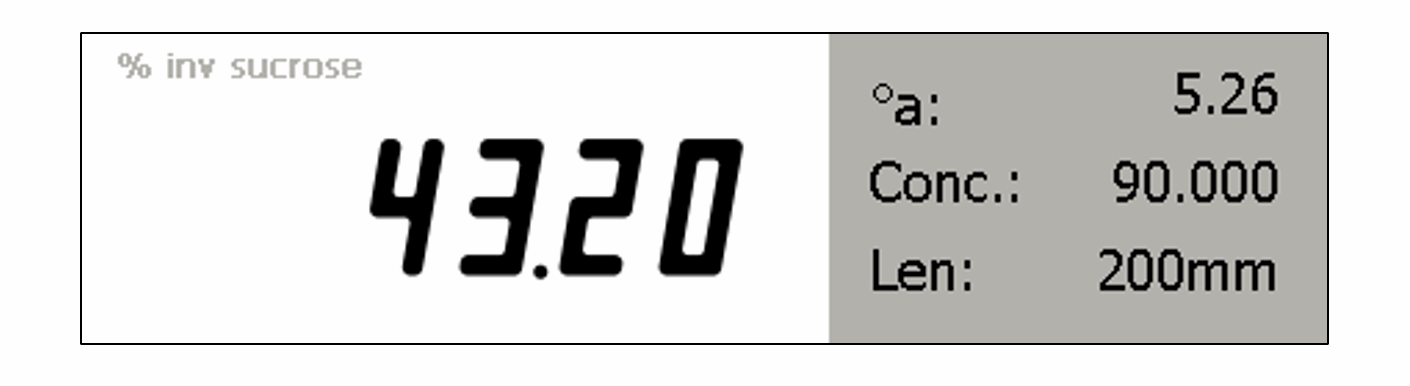
% Inversion of sucrose solution
The ADS400 Series Saccharimeter (or ADP Polarimeter) can automatically calculate % inversion of sucrose solution.
The Saccharimeter can measure the optical rotation (in °Angular) of a prepared sucrose solution; by telling the instrument the concentration of that solution, the instrument will reference known rotations in °A of non-inverted and fully inverted sucrose and then calculate the percent inversion of the sample being measured.
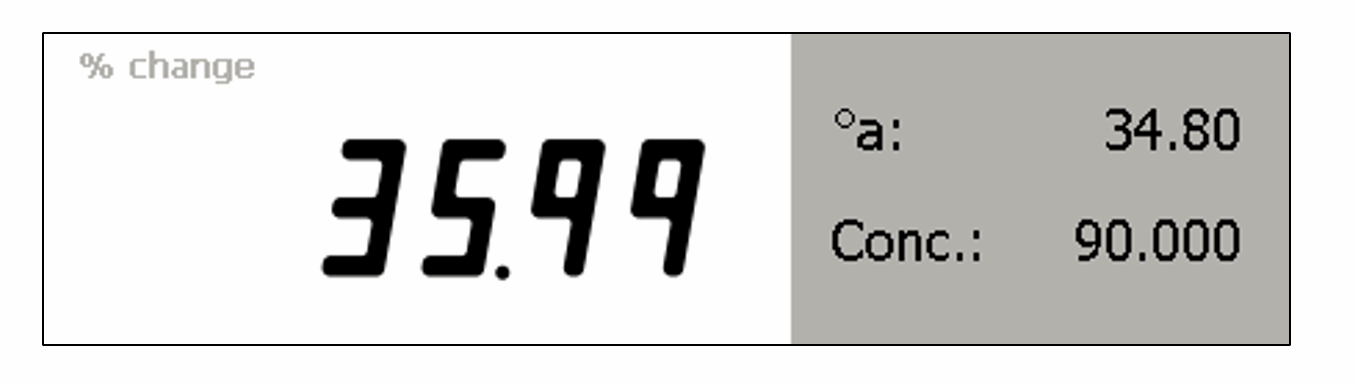
% Change of product over a period of time
It is not just sugar solutions that can change rotation values over time. A Bellingham + Stanley polarimeter can calculate a percent change for other chemicals.
By inputting known rotation values at the start and end of a reaction, and the concentration of a solution, the instrument can measure the current rotation and calculate the percentage change.
Digital Solutions for the Sugar Industry
Bellingham + Stanley’s digital polarimeters for the sugar industry, the ADS400 Series and ADS620P Saccharimeters are the ideal way to measure inversion.
To learn more about these products click here, or get in touch with our customer care team today.