1/27/2025
| Dr. Andreas Eich
Our expert will tell you everything you need to know about the Ubbelohde viscometer, which is available in different versions.
Functionality and advantages
 Ubbelohde viscometers, or suspended level viscometers, are characterized by a venting tube that allows pressure equalization between the atmosphere and the lower end of the capillary during measurement. This design ensures that the measuring liquid in the measuring bulb and capillary is mechanically decoupled from the residual liquid in the sample reservoir.
Ubbelohde viscometers, or suspended level viscometers, are characterized by a venting tube that allows pressure equalization between the atmosphere and the lower end of the capillary during measurement. This design ensures that the measuring liquid in the measuring bulb and capillary is mechanically decoupled from the residual liquid in the sample reservoir.
A decisive advantage of this design is that the filling quantity has no effect on the flow time, which makes it the preferred viscometer for polymer analytics in accordance with ISO 1628-1.
Different versions and applications
Ubbelohde viscometers are available in different versions, which are described in the Norm ISO 3105. Xylem produces the viscometer types of the SI Analytics® brand in accordance with DIN 53000-1 and ASTM D445. These include micro Ubbelohde viscometers for small sample volumes and special designs such as TC viscometers for automatic thermoelectric detection of opaque samples. These can be used with AVS® devices. There are also viscometers with a large reservoir for determining the intrinsic viscosity of polymers, in which dilution series can be prepared and measured immediately.
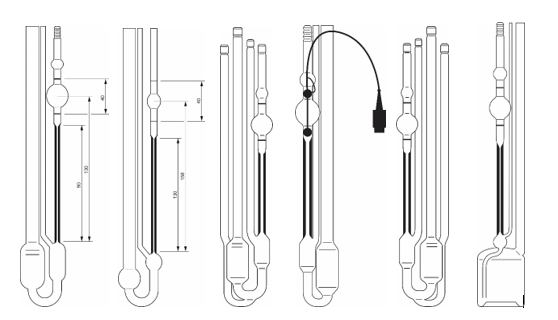
Figure 1: Various designs of Ubbelohde viscometers, for different applications.
Historical background
The name Ubbelohde viscometer traces back to Leo Ubbelohde (1877-1964), who applied for a patent for this type of device in 1932. Ubbelohde viscometers have been used as reference measuring instruments for viscosity measurement ever since due to their design-related accuracy advantages, e.g. also as national reference measuring instruments in metrological institutes.
Learn more about the Master Viscometer
Manual or automatic measurement of flow time
To measure the kinematic viscosity with capillary viscometers, the exact viscometer constant (calibration constant) is required, which is determined in the Xylem calibration laboratory for each individual viscometer. Xylem distinguishes between manual measurement of the flow time with a stopwatch and automatic measurement with light barriers in AVS® devices or ViscoClock plus. The flow times are not identical for manual and automatic measurement, because the ring measuring marks for manual measurement are not necessarily at the same height as the light barriers for automatic measurement. Accordingly, the calibration constants for manual and automatic measurement differ slightly.
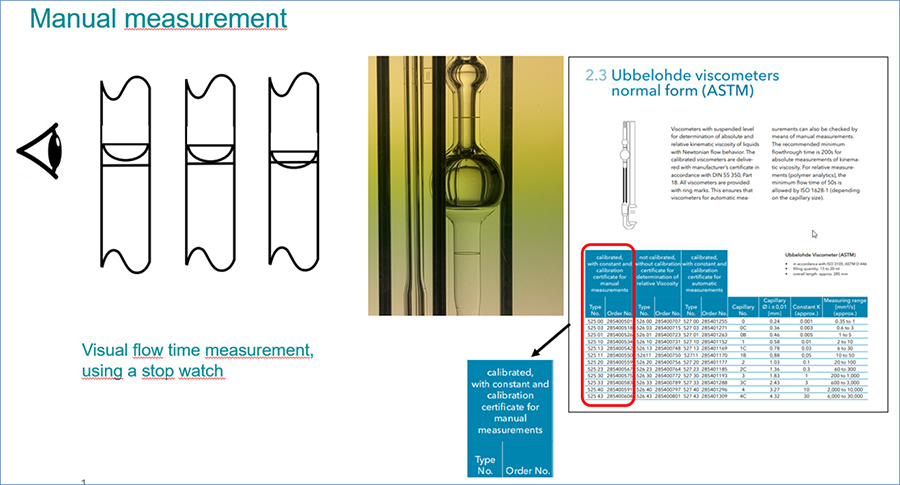
Figure 2: Manual measurement.
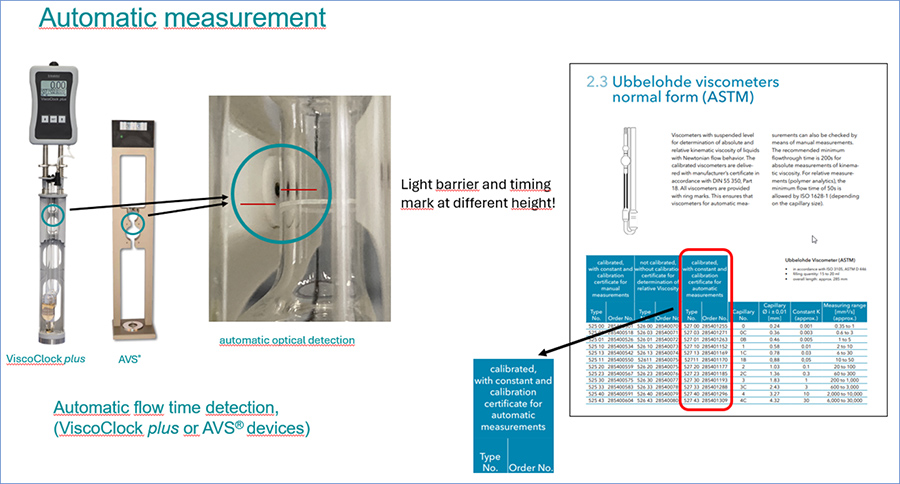
Figure 3: Automatic measurement.
In accordance with DIN 53000-1, viscometers that have been manually calibrated in our calibration laboratory have to be used for manual measurement, while for automatic measurement the calibration has to be carried out in AVS® devices, too. Accordingly, differently calibrated viscometers are offered by Xylem for each viscometer design.
To the products
An exception to this is polymer analytics, where relative viscosity and derived variables are usually determined. Here, the calibration constant is not required for the evaluation, so in principle, cheaper, non-calibrated viscometers can also be used. Nevertheless, the use of calibrated types is always recommended within QM systems to ensure traceability and quality monitoring.
Instructions for viscosity measurement
1. Preparation of viscometer:
-
-
- Make sure that the viscometer is clean and dry. Cleaning instructions can be found, for example, in Visco Handbook.
- For safe handling of Ubbelohde viscometers, a fixing bracket is recommended.
2. Filling the viscometer:
-
- Fill the sample into the sample reservoir of the viscometer via the filling tube.
- With highly viscous samples, make sure that there are no air bubbles in the capillary or the measuring sphere.
- Particles interfere with the flow through the capillary. Some samples may have to be filtered, especially with small capillary diameters of the viscometer.
3. Carrying out the measurement:
-
- Place the viscometer in a temperature control bath to stabilize the temperature of the sample. At room temperature, water with low lime content is recommended as the bath medium. Temperature stability is decisive for the accuracy of the viscosity measurement: Depending on the underlying measurement specification / standard, temperature uncertainties of only ±0.02 K to max. 0.1 K are permissible.
- The sample is pumped into the measuring sphere. For manual measurement, the easiest way is to aspirate the sample via the capillary tube, e.g. with a pipette ball. The venting tube must be closed during the pumping process.
- The venting tube is opened for the measurement. The flow time is measured between two ring marks for manual measurement or between the measuring levels of the detectors (light barriers or TC sensors) for automatic measurement (AVS® or ViscoClock device).
- The pumping up of the liquid and subsequent measurement of the flow time is repeated - typically three flow times are measured and their mean value is calculated.
4. Evaluation of measurement:
-
- The kinematic viscosity is calculated by multiplying the mean value of the flow time by the calibration constant of the viscometer.
- In polymer analytics, the flow time of the pure solvent is also required. The ratio of flow times, sample/solvent, gives the relative viscosity. Further quantities are derived from this result, e.g. the viscosity number or the intrinsic viscosity. The polymer concentration is also needed for their calculation.
- Make a note of the results and, if necessary, carry out several measurements to check the reproducibility.
5. Cleaning of viscometer:
-
- Clean the viscometer thoroughly after each measurement to avoid contamination.
- Store the viscometer in a safe place to prevent damage.

Content:
- Viscosity - Rheology
- Fundamental of capillary viscometry
- Measurement of the flow time
- Method fordetermining viscosity
- Calibration
- and more...
Here you can learn more about viscometry:
Blog: Use of Ubbelohde Master viscometers
Blog: FAQ Viscometry