What is FDA Regulation 21 CFR part 11?
Data integrity is important whatever line of work you’re in, but within the world of pharmaceutical manufacturing ensuring data integrity is a key component to complying with globally recognised standards.
Title 21 of the Code of Federal Regulations; part 11, Electronic Records; Electronic Signatures (also known as 21 CFR part 11), defines how electronic records, and associated electronic signatures on those records, can be submitted to the FDA (Food and Drug Administration). Whilst it is an American standard, it is widely followed globally, as pharmaceutical manufacturers who wish to sell into the US market will need to comply with its requirements.
Who needs to comply with FDA Regulation 21 CFR part 11?
All FDA-regulated industries, such as pharmaceutical, production of medical devices, food & beverage manufacturers and cosmetics companies, must comply with 21 CFR part 11. Computer systems (including analytical instruments with onboard computers) which store or produce data to make quality control decisions, or reporting data for the FDA must comply. This includes any laboratory results used to determine quality, safety, strength, efficacy, or purity. In manufacturing environments, data used to make decisions related to product release and product quality must also comply.
Which Xylem refractometers and polarimeters support FDA Regulation 21 CFR part 11?
Because 21 CFR 11 states that closed computer systems must have a collection of technological and procedural controls to protect data within the system, Bellingham + Stanley designed a selection of its instruments to help customers easily comply whilst using Bellingham + Stanley products. The current instruments that comply with 21 CFR part 11 are:
Polarimeters
- ADP450 Polarimeter
- ADP600 Series Polarimeters
Refractometers
- RFM900-T Series Refractometers
- RFM300-T Series Refractometers
How do these Xylem instruments comply with FDA Regulation 21 CFR part 11?
RFM900-T Series refractometers, ADP450 polarimeter & ADP600 Series polarimeters offer the following key benefits for 21 CFR part 11.
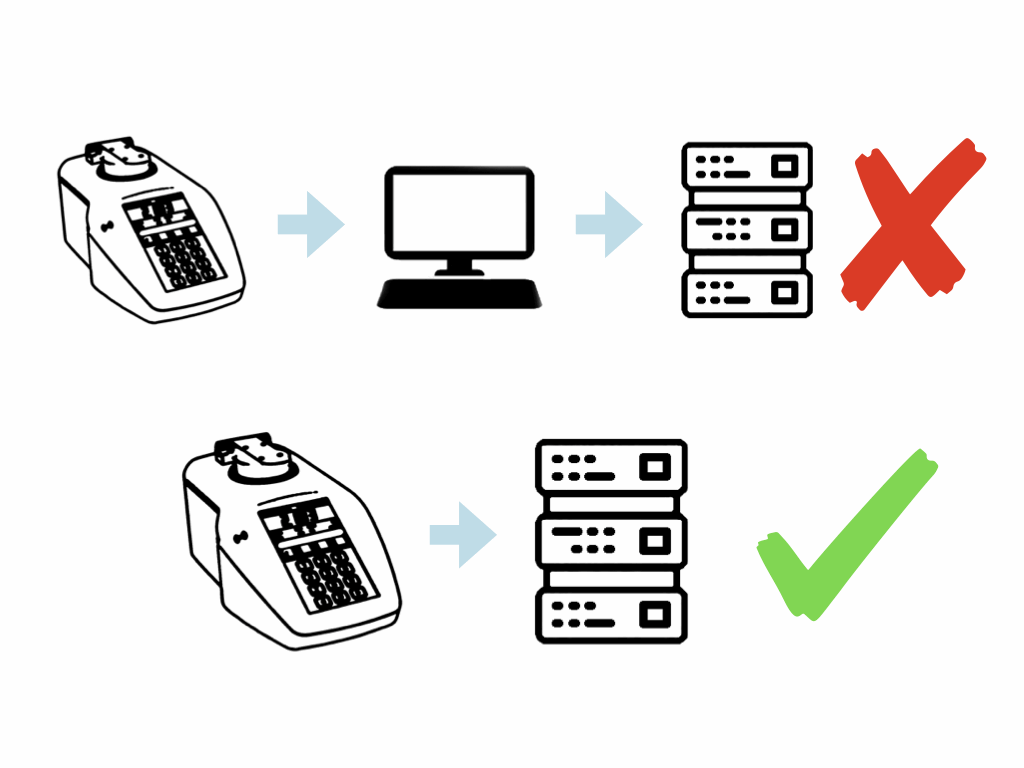
- Compliance without an intermediate PC – less data security risk and lower system cost
- Server synchronised clock to prevent data tampering
- Multi-reading PHR Method for batch measurement as per GLP/PHR
- Print to Secure PDF with custom header (customer logo can be input)
- Electronic signatures including multi-verification (Submitter > Reviewer >> Approver)
- XML output strings with encryption & MD5 check for easy connection to LIMS/Server
- Configurable users enforcing unique login & signature credentials as well as strict password controls
- Full instrument configuration audit trail
- Full validation documentation, service & support
The recording of results, and the logging of instrument access and configuration is also possible. The Review & Approve electronic signature process can even be achieved locally or remotely. Encrypted Print to Secure PDF, .csv and .XML outputs makes data integration to LIMS simple, secure and auditable.
ADP450 Series Polarimeters are the latest instrument to benefit from the 21 CFR part 11 feature set, with all ADP450 instruments manufactured after 2019 supporting the regulations as standard. For laboratories looking for even more accuracy in optical rotation measurement, or the ability to measuring at additional wavelengths, the flagship ADP600 Series polarimeters offer all of the above as well as 3-decimal place accuracy and a variety of pharmacopoeia cited wavelengths across the UV and visual spectrum. Available variants include: 325, 365, 405, 436 and 546nm as well as the 633nm wavelength – equivalent to a helium neon laser line. The ADP600 Series incorporates an onboard airflow Peltier temperature control system that stabilises the sample temperature at the pharmacopeia prescribed 20 or 25°C and now has the latest software for operation in FDA controlled environments. As such, the ADP600 Series meets all the requirements of pharmacopeia for optical and specific rotation (precision, wavelength, temperature), including the ability to measure Dextromethorphan Hydrobromide at 325nm, where readings must be within 1% of readings of a USP Reference Standard of the same material.
The same Method system incorporated in all ADP series polarimeters is also common across the RFM digital refractometer range too. This makes use of refractometer and polarimeter in the same laboratory very familiar to its operators. Connection to LIMS is made easy with a fully configurable XML data output making for simple integration with plant LIMS or generally available software packages including LogiLAB® SDMS and IKA laboratory technology’s Labworldsoft 6.
Refractometer software to help ensure data integrity
Let’s take a closer look at Bellingham + Stanley’s RFM900-T series of refractometers which come preloaded with many software features to help ensure compliance with 21 CFR part 11 right out of the box.
Subpart A of the regulation provides an overview of what the regulations apply to and provides definitions. Subpart B covers electronic records, and requirements for validation, security, authenticity of records and audit.
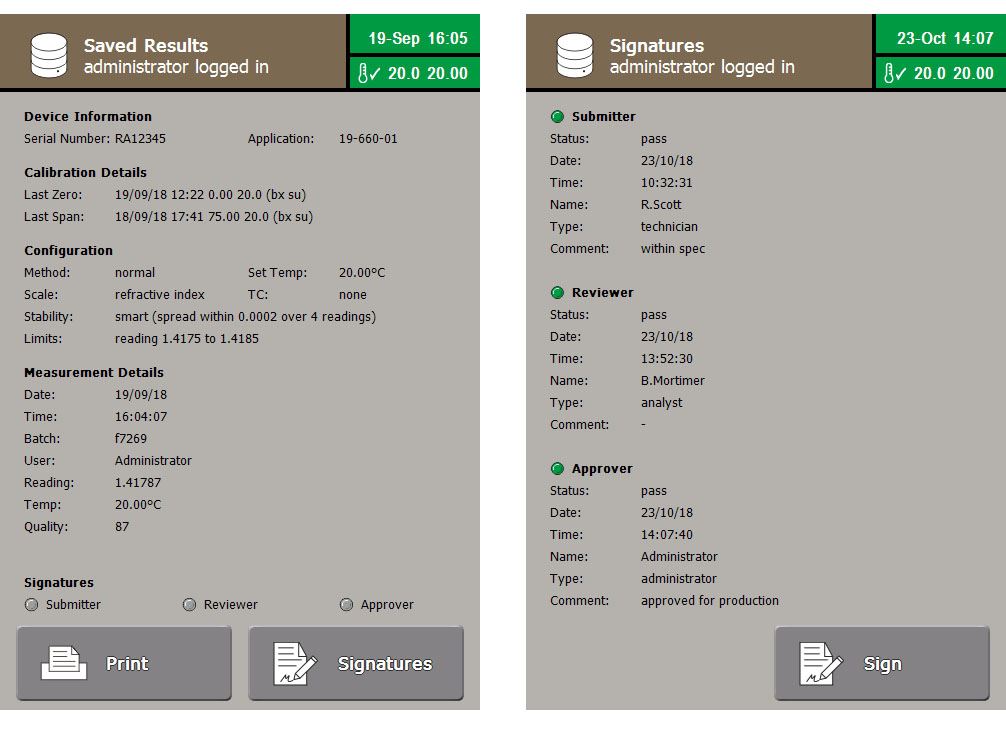
To help with this, the refractometers feature audit trails, which cannot be switched off, capable of storing up to 10,000 entries, as well as a separate reading log for another 10,000 reading results. Before any activity is performed the user must login with their username and password, this ensures all events are recorded with the name of the person performing it as well as the time and date. Logins are kept secure by demanding minimum password lengths, customisable to local requirements, and password complexity is ensured as the system can enforce the need for combinations of UPPER CASE and lower case letters, numbers and symbols.
Security is further assured by the ability to have passwords expire after a set period, the prevention of recycling of old passwords and locking users out if they incorrectly log in too many times.
Subpart C of 21 CFR part 11 focusses on electronic signatures, their use and application and controls to manage them.

The RFM900 Series of refractometers requires that both usernames and the signature associated with each user are unique, this ensures each signing can only be attributed to one user. When results are signed, not only is the user’s signature applied but that user’s role and the time and date of signing also recorded. Events in the system logs cannot be overwritten or altered. This ensures results are attributable, legible, contemporaneous, original and accurate (ALCOA).
User roles are fully definable, so access can be granted to any function of the instrument as applicable, whether a technician who is only given permission to perform readings, or an analyst who is granted access to the data logs to review data, whilst both are prevented from accessing system settings, such as clock adjustment or Method configuration. Their role also includes a signature level so they can sign as a submitter of a result, or a supervisor can later review and approve the final results.
Easy data transfer from refractometer to LIMS
Many companies will already operate a LIMS for long term storage of data. The RFM900-T Series of refractometers has been designed to easily transfer data from the instrument to a LIMS.
Records on the instrument can be generated in electronic form, as well as paper based. These electronic records can be saved to the instrument’s FTP memory. By connecting the refractometer to a network via its Ethernet port the files can be easily transferred to anywhere on the network or into the LIMS. This also avoids the use of USB memory sticks which can’t be used in many companies due to their insecure nature (although a USB option is still available if wanted!).
 Whenever a file is created on the instrument a checksum file is also created. This can be exported from the instrument at the same time as the main file, then using free Bellingham + Stanley software, “MD5 file verifier”, the checksum can be recalculated on the file and compared to the original thus ensuring that the file has maintained its integrity in the transfer and confirm that no one has subsequently altered it.
Whenever a file is created on the instrument a checksum file is also created. This can be exported from the instrument at the same time as the main file, then using free Bellingham + Stanley software, “MD5 file verifier”, the checksum can be recalculated on the file and compared to the original thus ensuring that the file has maintained its integrity in the transfer and confirm that no one has subsequently altered it.
The instrument logs can be easily filtered by date range, user, signed status, Method used and export status. This way the required data can be easily found, reviewed, signed and exported to LIMS. Transfer of logs to the FTP memory can also be automated so after a period of time logs will be exported in the background without the need for an operator to be present, and with free Bellingham + Stanley software “FTP instrument sync” those files placed anywhere on a network.
The ability to transfer files is limited to users who are granted access to the rights via their user role.
Traceable validation and calibration from UKAS accredited lab
Did you know… Bellingham + Stanley can also provide system validation (IQ, OQ, PQ) and traceable calibration standards from our UKAS accredited lab.
With additional features such as automated system backups, network user authentication and network-controlled instrument time, the RFM900 Series refractometers can provide everything needed to help operate in a 21 CFR part 11 environment.
Familiar software across a range of instrumentation
Don’t forget, the software features detailed above are available on a number of Bellingham + Stanley branded products including the RFM300-T Series refractometers, ADP450 polarimeters, and ADP600 Series polarimeters. No matter which model you choose, the fast and easy to use software will enable you to comply to FDA regulation 21 CF part 11.
For more information contact us today
For further reading on how Bellingham + Stanley can help you comply with FDA regulations 21 CFR part 11, take a look at our video on the same subject.
Ready to move to the next step? Contact us today and ask about Bellingham + Stanley polarimeters and refractometers. To read more about Bellingham + Stanley refractometers click here.
You can also request a customer information pack which includes instrument validation reports, software compliance reports and instrument connectivity reports but contacting our customer care team.
The refractometry guide with helpful hints and tips
 The refractometry handbook will teach you the basic principles of refractory and the instruments used to measure refractive index and °Brix. Originally written by pioneers of the handheld refractometer and revised over the years by experts in the field this handbook will guide you through different modes, the effects of temperature, sample prep and more.
The refractometry handbook will teach you the basic principles of refractory and the instruments used to measure refractive index and °Brix. Originally written by pioneers of the handheld refractometer and revised over the years by experts in the field this handbook will guide you through different modes, the effects of temperature, sample prep and more.
Download now