In this article, you will find answers to the following questions and topics related to pH measurement:
According to Sörensen, the pH value is the negative decadic logarithm of the activity of the hydrogen ions:
This is a somewhat unwieldy definition, but the following should shed some light on the matter. The pH value is an essential basis for a wealth of reactions in all water-based media and essential for us as living beings, but also in countless natural and technical contexts.
The concept of activity is most easily described as effective concentration. It can be visualized with the following image:
If someone tries to run through a dense crowd of people to reach a certain goal, they will probably have difficulty getting through. They will constantly be slowed down and have to change direction again and again.
It is the same with ions in a highly concentrated solution. Like the person running through a crowd, they also interact with other ions and the reaction proceeds more slowly. If, on the other hand, there are only a few people on the road, the runner gets through quickly and reaches his destination. The same is true for ions in a dilute solution. Thus, the activity increases, and it is easy to explain why the activity is chosen here to describe instead of the concentration: it indicates what can actually be measured.
Tip: With highly diluted solutions, we can always assume for simplicity's sake that concentration and activity are the same.
What values can the pH value assume?
Above it was already indicated that the pH value has to do with water. And it is indeed the case that water dissociateto a small extent into H+ ions and OH- ions.
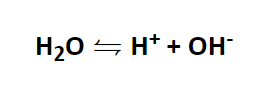
At exactly 25 °C, the so-called ion product is:
[H+] * [OH-] = 10-14
It follows from the two equations that the activity for H+ and OH- is the same and corresponds to 10-7 mol/l for both ions.
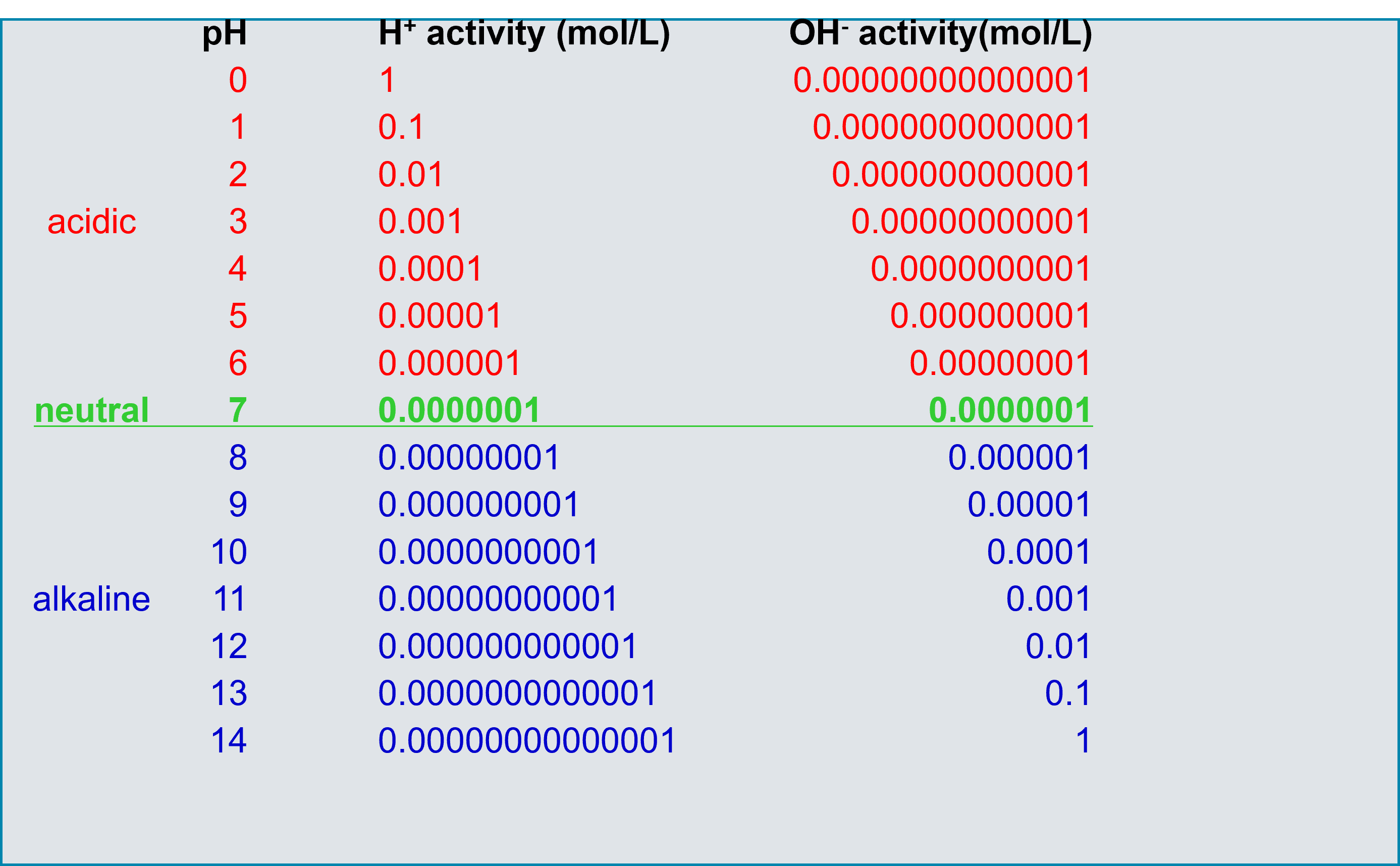
Fig. 1: Structure of a pH scale for determining the pH value.
All pH values lower than 7 show an increased activity of the hydrogen ion and are therefore acidic. When the activity of the hydrogen ion (proton) decreases, the activity of the hydroxide increases, the pH value becomes greater than 7 and the solution becomes basic. Technically, pH values greater than 14 and less than 0 are possible but cause great difficulties in terms of measurement.
What is the significance of the pH value?
As already indicated above, the pH value has a comprehensive significance in aqueous solutions and water-containing products. It influences chemical and biochemical reactions, is responsible for product properties, efficient presentation of chemical products and physiological processes.
Important needs of our daily life are fundamentally influenced by the pH value, for example, there are decided regulations about the pH range that our drinking water has. The pH value influences the color, taste, and shelf-life of our food. It is also measured to prevent damage to technology and nature and to be able to take countermeasures if necessary.
The graphic below gives an understanding of where we encounter the pH value in our everyday life:
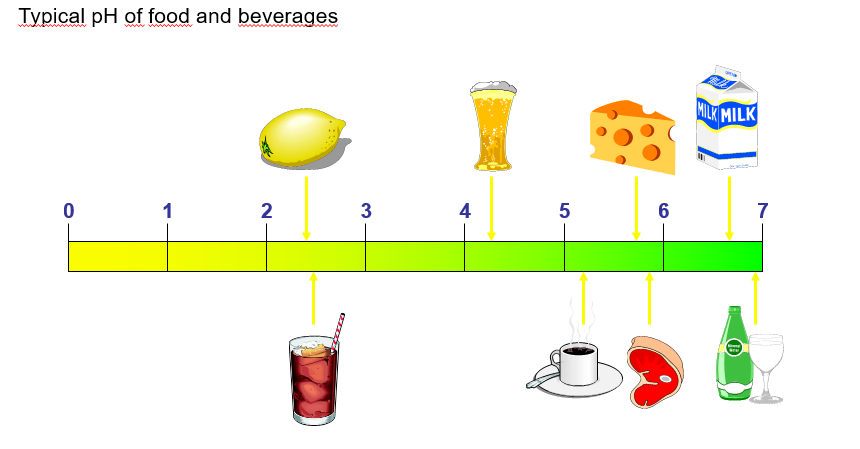
Fig. 2: Significance of the pH value in everyday life.
How is the pH value measured?
One of the most important organs for us humans, which "measures" the pH value on a daily basis, is our tongue. This important sense has crucial significance for us, as many foods are weakly acidic, suggesting edibility and freshness. Rotten or inedible things often come with a soapy taste that is perceived as disgusting and avoids consumption.
The drawback to the physiological sense of taste is that pH cannot be quantified. An example is cola drinks, where sugar or sweetener simply mask the pH of about 2.5 to 2.8 pH. Without this addition, we are in the range of pure household vinegar, which no one voluntarily drinks for refreshment!
There are many ways to measure pH. Indicator papers or color solutions are offered that change color depending on the pH.
The disadvantage is:
- the color reactions can be perceived differently
- the color change is too coarse
- they are not suitable for continuous measurement, for example when installed in a pipeline
In addition, a sample such as wall paint can make reading impossible.

pH Electrodes Selector
Are you wondering whether the pH electrode you have chosen is the right one?
Simply answer 5 questions in our new tool and you will receive the right pH electrode for your application.
Start now!
pH handbook - A Guide to pH Measurement
 In our pH handbook you will find our expert knowledge on the subject of pH/redox. Over 100 pages you will learn practical knowledge about pH measurement and pH determination. Numerous illustrations, tables and diagrams provide clear knowledge on how to measure the pH value correctly, calibrate the pH electrode, maintain it, clean it and many other helpful tips and tricks from our experts that will make your everyday work in the laboratory and in the field easier. Download the pH handbook as a PDF here.
In our pH handbook you will find our expert knowledge on the subject of pH/redox. Over 100 pages you will learn practical knowledge about pH measurement and pH determination. Numerous illustrations, tables and diagrams provide clear knowledge on how to measure the pH value correctly, calibrate the pH electrode, maintain it, clean it and many other helpful tips and tricks from our experts that will make your everyday work in the laboratory and in the field easier. Download the pH handbook as a PDF here.
The structure of a pH electrode
A pH electrode is an electrochemical sensor and is based on potentiometry, i.e. the measurement of a voltage. This voltage is described by the Nernst equation, the derivation of which is described in detail in all standard works of physical chemistry.
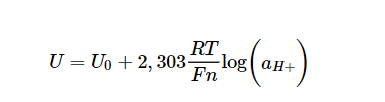
U: Potential of the pH electrode
U0: Asymmetry potential (e.g. at pH 7)
2.303: Conversion factor natural/decadic logarithm
R: General gas constant 8.3145 V As/(mol K)
T: absolute temperature in K
F: Faraday constant 9.648*104 As/mol
n: charge of the ion involved (H+, n=1)
log: decadic logarithm
a: activity (a = f * c), f: activity coefficient (0 < f ≤ 1).
In principle, the pH signal is a voltage signal caused by the activity of the hydrogen ions, measured in mV. The above equation is a straight-line equation with intercept (=asymmetry or zero point) and slope. If the above values are substituted into the slope term, a slope of 59.2 mV/pH at 298 K (= 25 °C) is obtained.
But what does the pH electrode look like now?
The pH electrode SenTix® 980 (often referred to as a glass electrode) consists of two components:
- The pH-sensitive part (glass electrode) the name given to the pH-sensitive special glass.
- The reference electrode, today almost exclusively a silver/silver chloride element embedded in a matrix, which provides a largely constant electrical potential against which the changing electrical potential of the glass electrode is measured.
There is also a reference element inside the glass electrode named the inner reference, which picks up the signal from the glass membrane. The electrical voltage signal is picked up at the terminal head and sent to the measuring instrument.
The following illustration shows the schematic structure in comparison with a commercially available pH electrode.
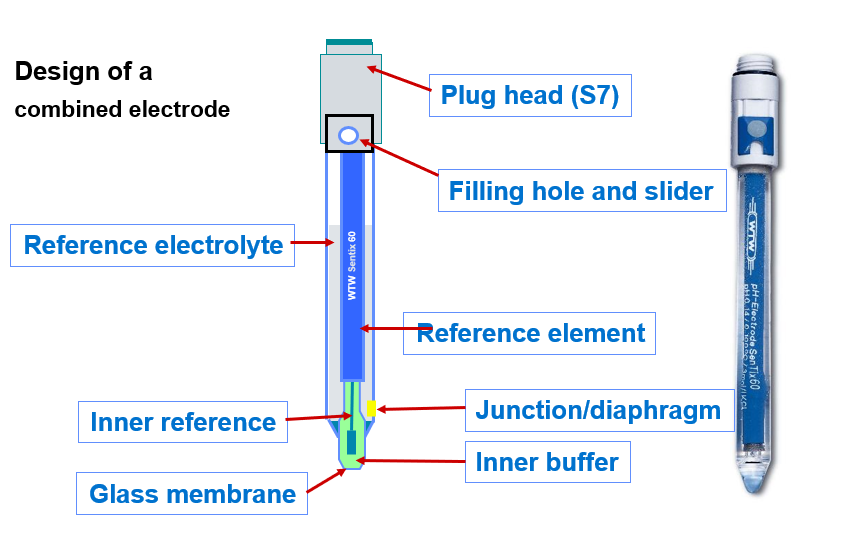
Fig. 3: Schematic structure of a pH electrode compared to a commercially available pH electrode.
Suitable pH electrodes for laboratory and field can be found here!
The pH meter or pH meter
A pH meter is in fact a high-impedance voltage meter. Depending on the application, they are available as laboratory instruments or in portable form for mobile use and also as stationary pH meters for measurement in processes.


Fig. 5: Portable pH meter ProfiLine pH 3310 with SenTix® 41.

Fig 6: Digital IDS pH electrode SenTix® 980.
 These pH measuring instruments can differ in their additional functions; these can be, graphic displays, memory functions, interfaces and other functions that support the work in the respective environment. In addition, there are also digital electrodes from the IDS (Intelligent Digital Sensors) Series, which digitize the measurement signal in the sensor head according to the same principle and transmit it to an evaluation device without interference.
These pH measuring instruments can differ in their additional functions; these can be, graphic displays, memory functions, interfaces and other functions that support the work in the respective environment. In addition, there are also digital electrodes from the IDS (Intelligent Digital Sensors) Series, which digitize the measurement signal in the sensor head according to the same principle and transmit it to an evaluation device without interference.
Request offer