7/5/2024
| Dr. Klaus Reithmayer
Here you can find out from our expert how best to measure the important parameter "dissolved oxygen".
 Dissolved oxygen is a reactive gas, which is used in the measurement. Until the beginning of the 60s of the 20th century, there was no simple solution for determining dissolved oxygen, a titration method was usually used, the so-called Winkler titration, which was published by Lajos Winkler in 1888. The dissolved oxygen was determined using a titration method using iodometry. It is a multi-stage process that can only be carried out under laboratory conditions with some effort and corresponding uncertainties.
Dissolved oxygen is a reactive gas, which is used in the measurement. Until the beginning of the 60s of the 20th century, there was no simple solution for determining dissolved oxygen, a titration method was usually used, the so-called Winkler titration, which was published by Lajos Winkler in 1888. The dissolved oxygen was determined using a titration method using iodometry. It is a multi-stage process that can only be carried out under laboratory conditions with some effort and corresponding uncertainties.
The family of electrochemical sensors
In 1962, Leland Clark (1918 – 2005) published the Clark electrode named after him, which is the mother of electrochemical oxygen sensors. This is an electrolyte-filled electrochemical cell separated from the medium to be examined by a gas permeable (Teflon®) membrane with an electrode system in which the dissolved oxygen on the surface of a cathode is reduced. The electrical current flowing in this process is a direct measure of the oxygen consumed and can therefore be used to measure it.
In this type of cell, the measuring device applies a defined voltage between the electrodes, at which this process runs optimally.
WTW® brand sensors, such as the TriOxmatic® family of sensors used in wastewater analysis, have worked and continue to work according to this principle. The name TriOxmatic® is derived from a third electrode that monitors the quality of the electrolyte used and gives a signal for its replacement.
Another type of dissolved oxygen sensor was developed at WTW® and is still widely used today, especially in the field, but also in laboratory applications: the CellOx® family of galvanic oxygen sensors.
The schematic drawing shows the design:
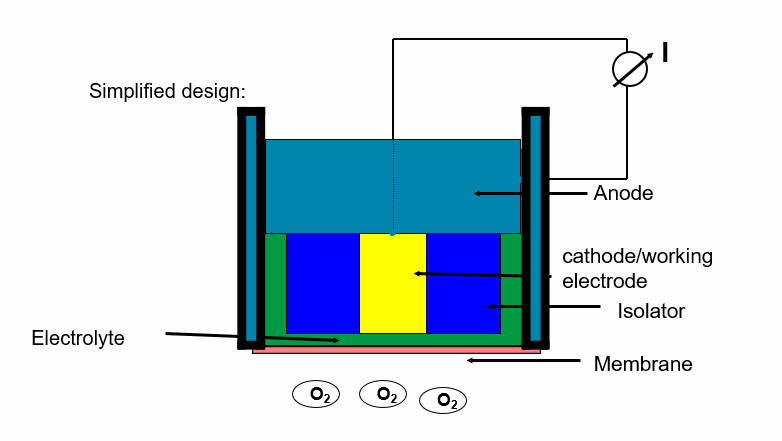 Fig. 1: Schematic drawing, electrolyte, anode, Isolator, cathode, Membrane
Fig. 1: Schematic drawing, electrolyte, anode, Isolator, cathode, Membrane
In contrast to polarographic oxygen sensors, these sensors have a kind of inner battery cell that generates the required voltage by itself: the cathode is made of gold, the anode of lead.
In the presence of oxygen, the following redox equation takes place:
2 Pb → 2 Pb2+ + 4e- Oxidation
4e- + O2 + 2 H2O → 4OH- Reduction
2 Pb + O2 + 2 H2O → 2 Pb(OH) 2 Overall reaction
This shows that these sensors need maintenance, because the precipitate from lead hydroxide has to be removed from time to time. However, the advantage is obvious, these sensors do not have to be connected to the measuring device either permanently or at least for a certain period of time like polarographic sensors in order to achieve their full functionality, but are practically ready for operation even when unplugged or switched on. The functionality of electrochemical oxygen sensors is also described in DIN EN ISO 5814.
Special features of electrochemical oxygen sensors
Electrochemical oxygen sensors, as you can see from the equation above, consume oxygen. This oxygen must be supplied. In the laboratory, it is necessary to stir, but for bodies of water such as rivers, streams or lakes, the natural flow is generally sufficient. If this is not guaranteed, the sensor consumes more oxygen than is supplied to it and there are lower readings.
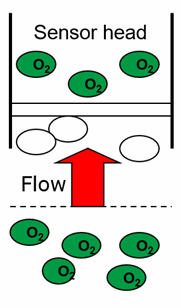
Fig. 2: Simplified representation of the flow direction of a galvanic oxygen sensor
Measurement with optodes
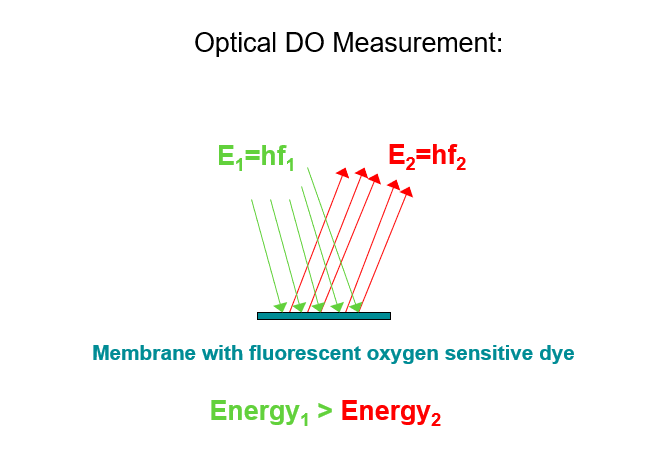
Fig. 3: Fluorescence: Intensity reduction and phase shift with increased oxygen concentration
In the 90s of the 20th century, the development of optical oxygen sensors, so-called optodes, initially began in medical applications for measuring respirometry. In the early 2000s, this type of measurement also reached the environmental market. The principle of this measurement is based on the so-called quenching, described by the Stern-Volmer equation. There are dyes that change into an excited state when irradiated with short-wave light and emit light in turn, this behavior is called fluorescence. The emitted light is significantly longer wavelength and therefore lower energy than the irradiated light.
This fluorescence of a suitable dye is reduced or extinguished depending on the concentration of a so-called quencher (in our case atmospheric oxygen).
F0 is the fluorescence intensity of the fluorescent dye in the absence of the quencher, F is the current intensity in the presence of the quencher, K is the Stern-Volmer constant, and [C] is the concentration of the quencher.
Phase shift between exciting and emitted light
In addition to the reduction in intensity, there is a much more stable behavior: As the concentration of the quencher increases, the phase angle between the irradiated and fluorescent light shifts:
Maximum intensity in the absence of oxygen
This method is now also an international standard and described in DIN ISO 17289-12 2014. At Xylem, both the FDO® 700 IQ, designed for the wastewater process, and the FDO® 925, designed for laboratory and field applications, work according to this principle.
Since optical oxygen sensors do not consume oxygen, stirring is not necessary in principle, but it supports the exchange of oxygen dissolved in the top layer, which protects the dye from damage.
Read more in our other blog articles about dissolved oxygen measurement: