7/12/2024
| Dr. Klaus Reithmayer
Here you can find out from our expert why and how electrochemical and optical oxygen sensors need to be calibrated.
Why do I need to calibrate?
There are several reasons why an oxygen sensor needs to be calibrated:

- Electrochemical sensors are based on the dissolution of metal electrodes in the presence of oxygen and, in interaction with the electrolyte, lead to a precipitation of poorly soluble salts. The precipitations cause increased internal resistance and thus reduced charge transport. This can be compensated electronically over a certain period.
- In the case of optical sensors, the dye "bleaches" over time and thus reduces fluorescence. This process can also be compensated for with the help of calibration and adjustment. The following applies here; If you can live with a low drift of a few percent (3 -5 %) per year, you can save yourself the calibration and with the factory correction factors ("factory calibration"), which are automatically transmitted via the sensor cap of WTW®sensors, a flawless measurement will be achieved.
How is an oxygen sensor calibrated?
Anyone hoping for a purchasable standard now will be pleasantly disappointed. For test purposes, there are gas mixtures that have a defined oxygen content. But: firstly, they are expensive and secondly, they are not necessary.
Only two variables play a role in calibration:
- The current oxygen partial pressure as a function of barometric, non-normalized air pressure
- The temperature-dependent water vapour partial pressure
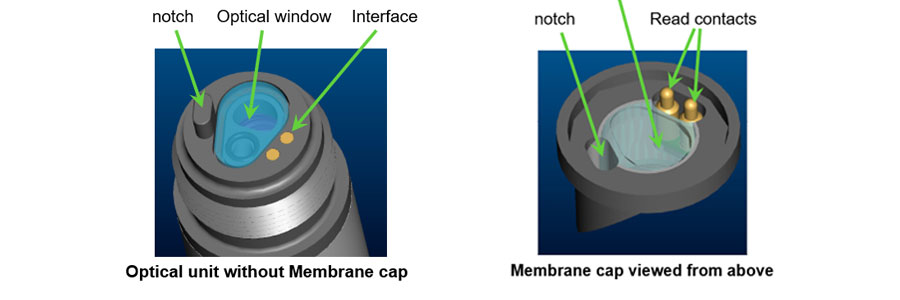
Fig. 1: View of the back and top of the membrane cap with read contacts and chip with calibration information
The proportion of oxygen in the regions generally accessible to us humans is always the same, namely around 21% of the air. It doesn't matter whether we are at sea level or at about 3800 m altitude on Lake Titicaca, the only change is the absolute air pressure and thus the corresponding partial pressure of the available oxygen. At sea level, it is around 212 hPa, at 3800 metres above sea level, it is at just under 650 hPa air pressure and only about 135 hPa. Of course, this must be considered during calibration.
The second important influencing factor is water vapour. The calibration is carried out in accordance with DIN EN ISO 5814:2013-02 in air saturated with water vapour, as these ratios can also be easily established in suitable vessels. From the known air pressure and the main temperature-dependent water vapour partial pressure at saturation (the pressure dependence can be neglected at "normal" altitudes above sea level), the current oxygen partial pressure can be calculated and used as a reference value. However, it is important that no water droplets are deposited on the membrane, as these lead to incorrect calibration results due to the different diffusion behavior from the gas and liquid phases.
In principle, all WTW®dissolved oxygen sensors are calibrated only in water vapour-saturated air, a zero-point calibration is not necessary. The sensors can be checked for a zero signal, using a sodium sulfite solution or a pure nitrogen atmosphere.
The calibration results
Electrochemical sensors
For all electrochemical sensors, a slope factor is determined internally, which leads to a valid calibration within a specified range. If the sensor is then tested in air, the result is a saturation value of 102 to 104 % air saturation. This over finding is normal, it is due to the fact that when immersed in liquid, an invariant layer of liquid is formed in front of the membrane due to the oxygen depletion caused by the sensor principle, which acts as an additional diffusion barrier and is of course not present in air.
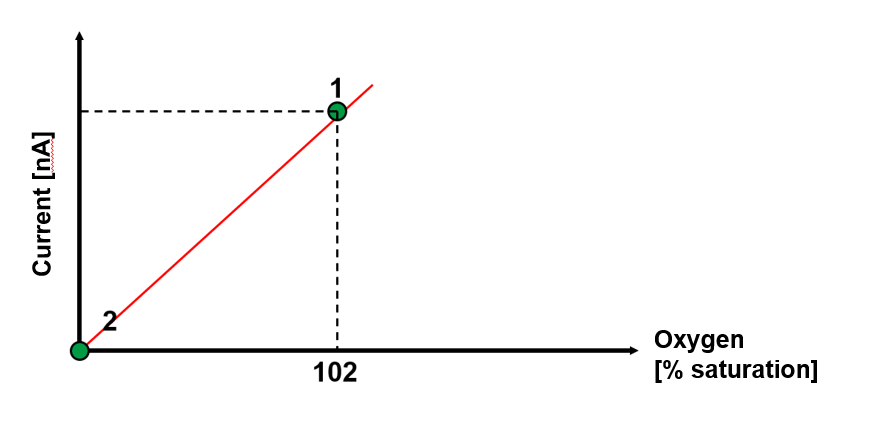
Fig. 2: Calibration curve of a galvanic oxygen sensor
The optical oxygen sensor
Electrochemical sensors show a linear characteristic curve during calibration, which arises from the amperometric principle. With optical sensors, the situation is somewhat different. Although quenching is not a linear process, a slope factor is also set here, but within narrower limits than with the electrochemical sensor. Since this type of sensor does not consume oxygen, the ideal value after calibration is 100% air saturation.
Read more in our other blog articles about dissolved oxygen measurement: