In addition to aqueous and non-aqueous pH titrations, there are many other titration applications where silver, platinum, double platinum and ion-selective electrodes are used, as well as a photometric sensor for all color change titrations.
What criteria do I now use to select the right electrode for my titration application?
The first and most important criterion is the type of titration application. As a rule, this results from the method description and the titrant used.
Example 1: If the titrant is silver nitrate, the application is a precipitation titration using a silver electrode with normal Ag/AgCl reference system AgCl 62 (see Fig. 1) or a silver electrode with a glass electrode as "reference electrode" AgCl 62 RG. RG means “Reference Glass”. In this case, there is no opening for refilling the electrolyte. See Fig. 2 AgCl 62:
Example 2: If the titrant is sodium thiosulfate, then it is a redox titration in which a platinum combination electrode such as the Pt 62 or Pt 61 is used.

Fig. 3: Pt 61
As with the silver combination electrode, there are also low-maintenance platinum combination electrodes with a glass electrode as the reference system. These RG electrodes can then always be used if the pH value does not change during the titration.
Digital IDS versions of the silver and platinum combination electrodes are also available, such as the Ag 62 IDS and Pt 62 RG ID.

Fig. 4: KF 110
For applications with polarizable electrodes such as SO2 titration in wine, determination of the bromine number and Karl Fischer ("KF") titration, there are double platinum electrodes such as the Pt 1200 and the KF 1100. See Fig. 4 KF 1100:
An overview of the applications and the corresponding electrodes can be found in Table Applications.
| Applications |
Recommended electrodes for Titration |
|
|
Applications
|
Details
|
Type of Electrode
|
Order number
|
|
Precipitation titrations
|
Titrations with silver nitrate - also back titrations with ammonium thiocyanate, sodium or potassium chloride etc. .
|
General chloride, chloride/NaCl ("salt") in foodstuffs
|
AgCl 62
AgCl 62 RG
Ag 62 IDS
|
285102413
285102100
285102150
|
|
Precipitation titrations
|
Titrations with silver nitrate - also back titrations with ammonium thiocyanate, sodium or potassium chloride etc. .
|
Cyanide, bromide, iodide
|
Ag 6280
Ag 62 RG
Ag 62 IDS
|
285102343
285102090
285102150
|
|
Precipitation titrations
|
Titrations with silver nitrate - also back titrations with ammonium thiocyanate, sodium or potassium chloride etc.
|
Mercaptans and hydrogen sulfide, cyanide
|
AgS 62 RG
Ag 1100 + A 1180
|
285102110
285103607 + 1057997
|
|
Precipitation titrations
|
Titrations with lanthanum nitrate
|
Fluoride
|
F 1100 PLH + reference electrode
|
285216295
|
|
Precipitation titrations
|
Titrations with Hyamine,
Cetylpyridinium chloride,
Sodium dodecyl sulfate, Sodium tetraphenylborate
|
Anionic, cationic and non-ionic surfactants
|
TEN 1100 + reference electrode
|
285096980
|
|
Redox titrations
|
Titrations with iodine and sodium thiosulfate (iodometry), potassium permanganate, cerium(IV) sulfate, potassium dichromate, ferrous (II) sulfate
|
Iodine value, peroxide value, chlorine, hypochlorite, potassium permanganate, etc.
|
Pt 61
Pt 62
|
285102019
285102002
|
|
Redox titrations
|
Titrations with iodine and sodium thiosulfate (iodometry), potassium permanganate, cerium(IV) sulfate, potassium dichromate, ferrous (II) sulfate
|
Oxidizability, H2O2, oxygen according to Winkler
|
Pt 62 RG
|
285102070
|
|
Redox titrations
|
Titrations with iodine and sodium thiosulfate (iodometry), potassium permanganate, cerium(IV) sulfate, potassium dichromate, ferrous (II) sulfate
|
Nitrate
|
Pt 62 RG IDS
|
285102140
|
|
Redox titrations
|
Titrations with ammonium iron(II) sulfate
|
COD
COD with sample changer
|
Pt 61
Pt 5901
|
285102002
285105065
|
|
Redox titrations
|
Titrations with iodine and polarizable electrode (dead stop)
|
SO2, Bromine number, etc.
|
Pt 1200
|
285103512
|
|
Redox titrations
|
Volumetric KF-titration
|
|
KF 1100
|
285102030
|
|
Redox titrations
|
Coulometrische KF-Titration
|
(Indicator electrode only)
|
KF 1150
|
285102060
|
Except for the KF 1100 and KF 1150, all electrodes have a plug head. A suitable coaxial electrode cable such as the L 1 A or L 2 A (Fig. 5 coaxial electrode cable L 1 A/L 2 A) is also required.
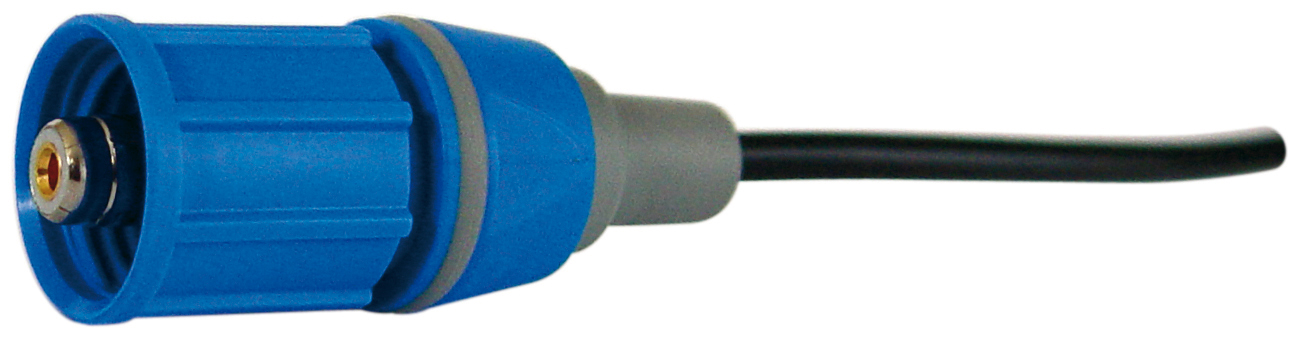
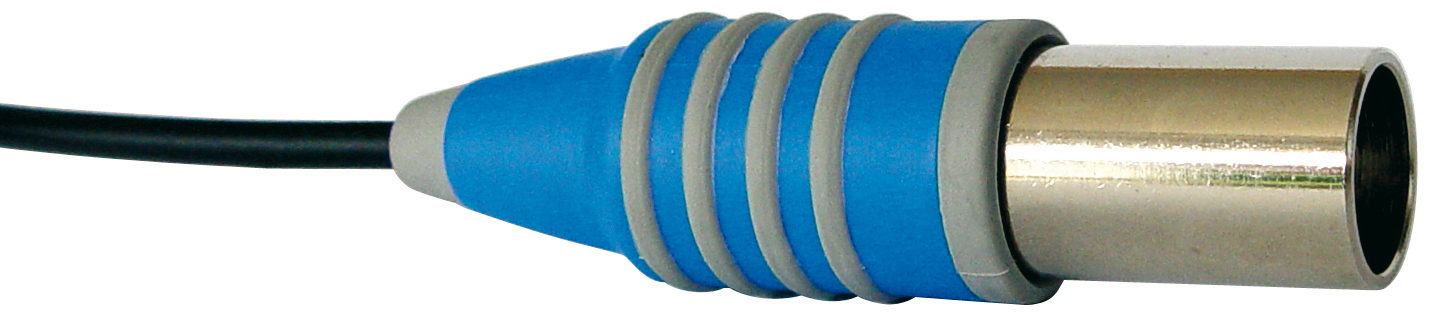
Fig. 5: coaxial electrode cable L 1 A/L 2 A
OptiLine 6, one sensor for all titrations on color change

Fig. 6: Optiline 6
For all titrations that are (have to be) performed on color change, you can use the OptiLine 6 digital photometric sensor (see Fig. 6 Optiline 6). The OptiLine 6 is connected via USB to the TL 7XXX titrators, whereby the wavelength used during the titration (= 6, 470 - 625 nm) is defined within the titration method. Due to the additional BNC and DIN connector, the OptiLine 6 can also be connected to older titrators, as well as to the TitroLine® 5000. The wavelength and other settings can then be preset via PC software.
Further information can be found in the titration catalog

Find here all components for a manual or automated titration with our high-precision software Titri Soft 3.5 for your use in the laboratory. Learn more about all products in the laboratory environment: coulometric and volumetric titrators, burettes, sample changers, the matching software and the corresponding titration electrodes and all accessories around titration.
Learn more
Find answers in the comprehensive titration handbook
 This is an excerpt from the titration handbook. On 192 pages it offers a compact introduction to the theory and practice of titration. If you are interested, you are welcome to download the practical guide as a PDF or request it as a brochure. And in our database you will find numerous applications for your titration for download. A concrete application example of the determination of acid in bases is described in the blogarticle "how to get correct and reproducible results in titration?". Our titration expert answers further questions from our customers in the blog article FAQ on titration.
This is an excerpt from the titration handbook. On 192 pages it offers a compact introduction to the theory and practice of titration. If you are interested, you are welcome to download the practical guide as a PDF or request it as a brochure. And in our database you will find numerous applications for your titration for download. A concrete application example of the determination of acid in bases is described in the blogarticle "how to get correct and reproducible results in titration?". Our titration expert answers further questions from our customers in the blog article FAQ on titration.
Further questions are answered by our expert in the blog article FAQ Titration. Helpful tips for your application area, you can read in our other blog articles: