We know that HCl and NaOH react in the ratio 1/1 and therefore 1 ml of a NaOH solution corresponds to 0.1 mol/l = 1 ml of a HCl solution 0.1 mol/l. That is 3.64 mg HCl.
Since the sample contains approx. 3.5 - 5 % hydrochloric acid, there are then between 3.5 - 5 g acid in 100 g sample. In 1 g sample this would be 35 - 50 mg HCl. In our example, the titrator has an exchange unit/burette with a volume of 20 ml. The optimal range for a 20 ml burette is 50-75 % of the nominal volume. So here this is 10 - 15 ml titrant consumption.
35 - 50 mg HCl (35/3,64 und 50/3,64) entsprechen einem Verbrauch von 9,6 - 13,7 ml einer NaOH 0,1 mol/l. Mit einer Probenmenge von etwas mehr als 1 g wären wir also in unserem Beispiel im optimalen Bereich.
It is correct that the concentration of ready-made solutions should then really be 0.100. Manufacturers indicate, for example, a titre of 1.000 with 0.02 %. So between 0.9998 and 1.0002 or between 0.09998 and 0.10002 mol/l.
Caution: But bases absorb CO2 from the air very quickly. This changes the titer and leads to false and non-reproducible results.
You can prevent the absorption of CO2 by adding a CO2 absorbent such as soda lime, a mixture of sodium and calcium hydroxide, to the "dry tube".
First put some glass wool or paper in the tube and then fill it up with soda lime. Change the absorbent monthly or when an indicator, if present, responds accordingly.
Find answers in the comprehensive titration handbook
 This is an excerpt from the titration handbook. On 192 pages it offers a compact introduction to the theory and practice of titration. If you are interested, you are welcome to download the practical guide as a PDF or request it as a brochure. And in our database you will find numerous applications for your titration for download. A concrete application example of the determination of acid in bases is described in the blogarticle "how to get correct and reproducible results in titration?". Our titration expert answers further questions from our customers in the blog article FAQ on titration.
This is an excerpt from the titration handbook. On 192 pages it offers a compact introduction to the theory and practice of titration. If you are interested, you are welcome to download the practical guide as a PDF or request it as a brochure. And in our database you will find numerous applications for your titration for download. A concrete application example of the determination of acid in bases is described in the blogarticle "how to get correct and reproducible results in titration?". Our titration expert answers further questions from our customers in the blog article FAQ on titration.
What other advantages does the titer determination of the titrant offer besides the exact determination of the concentration:
- Checking the electrode used: setting behavior, what does the titration curve look like, etc.
- Verification of the titration system as a whole with a certified volumetric standard.
- Correct any dosing deviations of the titration system.
Certified volumetric standards are used for titration. The certified standards are secondary standards whose concentrations in turn can be traced back to primary standards of national metrological institutes such as PTB (Physikalisch-Technische Bundesanstalt) or NIST (National Institute of Standards and Technology).
Table 1 shows the most common volumetric standards for the various titration solutions.
Titrationstyp
|
Standard
|
Alkalimetry
|
Potassium hydrogen phthalate, benzoic acid
|
Acidimetry
|
"TRIS" = Tris hydroxymethyl-aminomethane,
sodium carbonate,
Potassium hydrogen phthalate for non-aqueous titrations with perchloric acid
|
Argentometry
|
Sodium chloride
|
Iodometry
|
Potassium iodate, arsenic trioxide (rather rare today)
|
Cerimetry, manganometry
|
Sodium oxalate, iron (II) ethylene diammonium sulphate
|
Complexometry
Table 1: Volumetric standards |
Calcium carbonate,
Zinc
|
Which type of balance do you need for an accurate weighing of 0.2 - 0.2 g of the volumetric standard?
Potassium hydrogen phthalate is used for the titration of NaOH 0.1 mol/l. This standard is dried for two hours at 105 °C in a drying oven before use. For a titrant consumption of 10 - 15 ml weigh out 0.2 - 0.3 g accurately on a laboratory balance.
- Only which laboratory balance should it be for an exact weighing-in of 0.2 - 0.3 g?
- Is a precision balance with a readability/resolution of 1 mg (0.001 g) sufficient?
- Or should it better be an analytical balance with 0.1 or even with 0.01 mg resolution?
- How great is the influence of the uncertainty in weighing on the titration result?
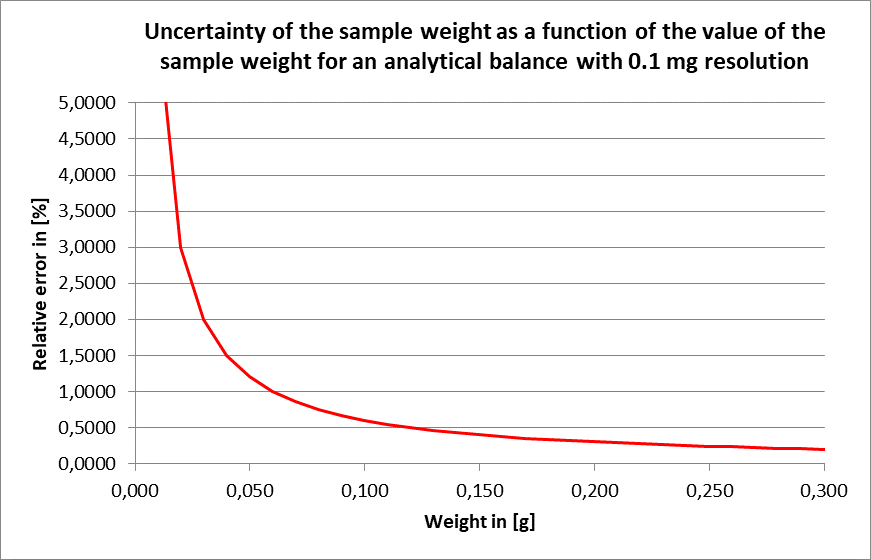
Fig. 1 Uncertainty of the sample weight as a function of the value of the sample weight for an analytical balance with 0.1 mg resolution.
The graph shows the uncertainty of weighing with the systematic and random components for an analytical balance with 0.1 mg resolution. A sample weight of 0.2 to 0.3 g could contribute to an error between 0.2 - 0.3 %.
This is acceptable, but also shows that a precision balance would not be sufficient, since here the error would be a factor of 10 higher, i.e. 2 - 3 %.
For initial weights < 0.1 g, ideally use an analytical balance with 0.01 mg resolution, otherwise the weighing error will have too great an effect on the result.
What other measures should be taken into account before performing titer determination and sample titration?
Before you start carrying out the titer determination and sample titration, you should take a closer look at the titrator and the electrode.
The following three measures are recommended:
- Rinse the exchange unit/burette to ensure that there are no air bubbles in the system. Never rinse back the system. Above all, this should not be done with alkaline solutions such as NaOH, as air and thus CO2 could get into the titration solution.
- The refill opening of the pH electrode is opened. If there is too little electrolyte in the electrode, it is refilled (KCl 3 mol/l). Important: the level of the electrolyte must always be above the sample solution during use, otherwise the sample solution can be pressed through the diaphragm into the electrode and possibly "poison" the electrode.
- We also recommend performing a pH calibration. Not because the one exact pH value is important here for the two applications, but a pH calibration is still the best way to check the quality of the pH electrode.
- The position of the electrode in relation to the titration tip in the titration clamp is very important. The electrode should be as far away as possible from the inlet of the titration tip so that the titration solution is always well mixed with the sample solution before it hits the electrode. See the arrangement at the bottom of Fig. 2. The stirring direction here is clockwise.
- The electrode should also be immersed deep enough in the solution so that the diaphragm is also inside the solution. Of course, the titration tip should also be immersed in the solution, otherwise too large drops will form during the titration and fall irregularly into the titration solution, resulting in unsteady titration curves. See the immersion depth of the pH electrode and the titration tip at the bottom of Fig. 2.
- The stirring speed should be set as fast as possible. Preferably so that a small funnel forms without creating air bubbles. See the funnel at the bottom oft eh solution on Fig. 2.

Fig. 2: Electrode and titration tip immersed in the sample solution.
Recommendations for carrying out the titer determination
If you follow all the tips, you will usually achieve good and reproducible results:
- The method of titer determination for NaOH is available in our TitroLine ® titrators and in our TitriSoft software as a standard method. It can be called up/loaded and used directly without changing the parameters if a triple determination is to be carried out.
- The potassium hydrogen phthalate standards weighed out in 100 or 150 ml beakers are filled up to 60/80 ml with deionised CO2-free water and completely dissolved.
- Immerse electrode and titration tip as described above and start titration.
- For titer determination, a threefold determination is the minimum. More determinations are not an error. In addition to the result, the titrator or the titrator software will also output the mean value and the relative standard deviation (from three determinations). The relative standard deviation is the measure of how the individual values deviate from the mean value.
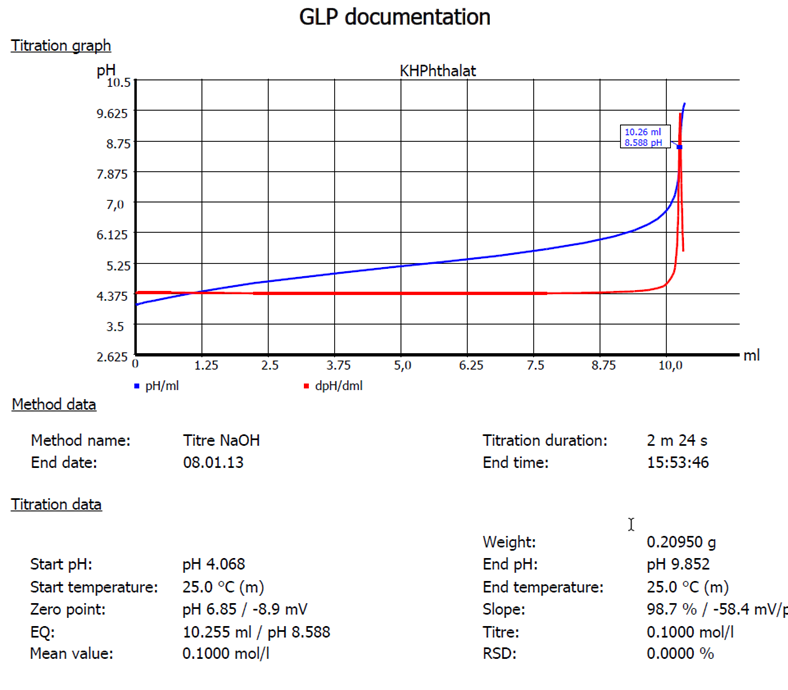
Fig. 3: Titration of potassium hydrogen phthalate with NaOH 0.1 mol/l.
Carrying out the sample titration

After the titer has been determined, nothing more stands in the way of titrating the acid sample:
- Weigh approx. 1 g sample into a 100/150 ml beaker and fill up to 60/80 ml with deionized water.
- Immerse electrode and titration tip as described above and start titration.
- For the titration of the strong acid HCl there is also a standard method in our TitroLine ® titrators and in our TitriSoft software under "pH strong acid". Only the calculation has to be adapted to HCl in % and can then be saved e.g. as a new user method.
The first titration curve looks as it should (Fig. 4). There is a clear equivalence point indicating the titrant consumption of the NaOH. This is what the titration curve of a strong acid like hydrochloric acid titrated with a strong alkali like NaOH and containing no CO2 or carbonate looks like. The second titration curve (Fig. 5) shows two equivalence points because part of the NaOH has converted to Na2CO3. This means that part of the titration solution is now Na2CO3 and reacts accordingly with the HCl according to the following reaction equation.
Na2CO3 + 2 HCl -> 2 NaCl + H2O + CO2
The titration solution is now no longer NaOH, but a mixture of NaOH + Na2CO3.

Fig 4.: Titration HCl with NaOH. NaOH contains no or only very little carbonate.

Fig. 5: Titration HCl with NaOH containing carbonate.
If the titrator is to titrate to an equivalence point, it may happen that the titrator stops titrating after the first equivalence point has been recognised. But it can also happen that the titrator stops titrating after the second equivalence point. This then leads to clearly different, non-reproducible results.
Carbonate in alkaline titrants such as sodium hydroxide or potassium hydroxide is, according to our many years of experience, the most frequent error in titration.
Using a concrete task, we tried to show how you can achieve correct and reproducible results through simple and comprehensible measures. The task seemed simple, but you could see that one or more errors can quickly lead to incorrect and non-reproducible results.
We have not gone into sample preparation in more detail in this blog. A liquid homogeneous sample containing max. 5 % hydrochloric acid can be titrated by weighing in without further sample preparation.
Further information can be found in the titration catalog

Find here all components for a manual or automated titration with our high-precision software Titri Soft 3.5 for your use in the laboratory. Learn more about all products in the laboratory environment: coulometric and volumetric titrators, burettes, sample changers, the matching software and the corresponding titration electrodes and all accessories around titration.
Learn more
Find answers in the comprehensive titration handbook
 This is an excerpt from the titration handbook. On 192 pages it offers a compact introduction to the theory and practice of titration. If you are interested, you are welcome to download the practical guide as a PDF or request it as a brochure. And in our database you will find numerous applications for your titration for download. A concrete application example of the determination of acid in bases is described in the blogarticle "how to get correct and reproducible results in titration?". Our titration expert answers further questions from our customers in the blog article FAQ on titration.
This is an excerpt from the titration handbook. On 192 pages it offers a compact introduction to the theory and practice of titration. If you are interested, you are welcome to download the practical guide as a PDF or request it as a brochure. And in our database you will find numerous applications for your titration for download. A concrete application example of the determination of acid in bases is described in the blogarticle "how to get correct and reproducible results in titration?". Our titration expert answers further questions from our customers in the blog article FAQ on titration.
Further questions are answered by our expert in the blog article FAQ Titration. Helpful tips for your application area, you can read in our other blog articles: